iso 8655 pipette calibration frequency|iso 8655 2022 pdf : traders ISO 8655:2022 and Sartorius Solutions for Pipette Calibration ISO 8655:2022 is an essential update for laboratories looking to ensure the accuracy and reliability of their volumetric . This document discusses the updates to ISO 8655:2022 and their impact on pipette calibration. It highlights the changes made to improve the accuracy of POVAs .
+ le traitement autoclave Classe 4 garantit la pérennité des lames dans le temps. + la teinte Verte s'estompe auprès 4 mois d'exposition au soleil. Elle est alors remplacer par une belle teinte .
{plog:ftitle_list}
These tests are designed to show that the sterilizer chamber does not leak in empty chamber. Leakage of air into the chamber is not acceptable for two reasons: The presence of air inhibits .
Information in the ISO 8655 includes standards for maximum permitted errors, guidelines for how to test and calibrate pipettes, reporting requirements to the end user, and how the end user can maintain their pipettes.The main changes are as follows: — general requirements for frequency of calibration, .ISO 8655-1 Second edition 2022-04 Reference number ISO 8655-1:2022(E) . — general requirements for frequency of calibration, reporting measurement errors, exchangeable parts, metrological confirmation, routine testing, and maintenance and repair, suitability of . ISO 8655-2, Piston-operated volumetric apparatus — Part 2: Pipettes ISO .Revised ISO 8655 has ten parts with stricter guidelines about pipettes, errors, and user info. Pipette testing and calibration are in Parts 6, 7, and 8, with high-precision balance requirements. Sartorius has decoded the standard and shares its expertise on in-house or outsourcing pipette calibration, covering balances, pipettes, tips, and related services.
Pipette Service and Calibration (ISO 8655 and ISO 17025) Installation Services . Qualification Services . Preventive Maintenance Services . Calibration Services . . 16-channel pipette: 24-channel pipette: ISO 8655 Verified: 4 per test volume* 1: 10 per test volume* 1: included : 0082 000 234 . 0082 000 235 . 0082 000 236 . 0082 000 237 .ISO 8655:2022 and Sartorius Solutions for Pipette Calibration ISO 8655:2022 is an essential update for laboratories looking to ensure the accuracy and reliability of their volumetric . This document discusses the updates to ISO 8655:2022 and their impact on pipette calibration. It highlights the changes made to improve the accuracy of POVAs .
ISO 8655 is the official standard for piston-operated volumetric apparatuses, which includes pipettes, burettes, dilutors, dispensers, and manually operated precision laboratory syringes. An updated version of the standard came into force in May 2022 and included the following significant changes for pipettes: The new ISO 8655:2022 has nine parts The ISO 8655:2022 for pipette calibration has nine parts; that’s two more than the previous version. . Stricter language about pipettes: Part 2 ISO 8655 Part 2 specifies maximum permissible errors for pipettes, requirements for markings, and the information manufacturers must provide. “Part 2 includes .

Best Practices for ISO 8655:2022 Compliance. To achieve and maintain compliance with ISO 8655:2022, laboratories should adopt the following best practices: Regular Calibration: Establish a routine calibration schedule based on the frequency of pipette use and the criticality of the measurements. Regular calibration helps in identifying and . The recommended frequency is based on the risks associated with using the POVA. The maintenance interval is affected by such factors as pipetting frequency, liquids dispensed and the age/model of the pipette. . 18. What is the difference between an ISO 8655 and an accredited ISO 17025 pipette calibration? In short, ISO 8655 describes how the .There are a number of different standards that guide and define the pipette calibration industry. Among the most important are ISO/IEC 17025:2017 and ISO 8655, which each define separate aspects of a quality calibration.It’s important to understand that neither standard alone is sufficient: compliance to both is required for accurate and reliable pipette calibration.
We expect pipettes to accurately dispense liquid but after a while, pipettes will need calibration. The type of calibration your pipettes need may vary depending on usage, quality systems, and more. There are two primary types of ISO pipette calibration, which are known as ISO 8655 and ISO 17025.ISO 8655 is the official standard for piston-operated volumetric apparatuses, which includes pipettes, burettes, dilutors, dispensers, and manually operated precision laboratory syringes. An updated version of the standard came into force in May 2022 and included the following significant changes for pipettes:ISO 8655:2022, Important Changes for Pipette Calibration. Free Guide: ISO 8655 Compliance, Key Points for Pipette Calibration; The Challenges of the Pipette Calibration Workflow; . Routine testing and calibration: Frequency. 1x/day – 1x/month. Routine testing: 1x/day – 1x/month, calibration: 1-4x/year (according to risk assessment .
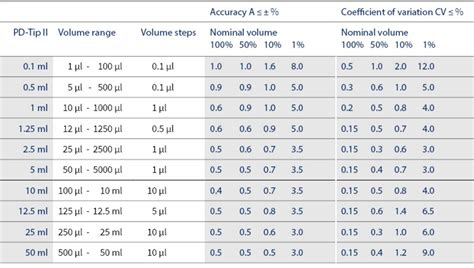
6.4 Test frequency. 6.5 Exchangeable Parts. 7 Routine testing. 8 Determination of pass/fail status. . Guidelines on the calibration of non-automatic weighing instruments [8] . ISO 8655-2:2020, Piston-operated volumetric apparatus — Part 2: Pipettes; ISO 8655-3:2020, Piston-operated volumetric apparatus — Part 3: Burettes;International standard ISO 8655 for POVA devices . The required time interval should be based on the frequency of use, number of operators using the device, nature of the liquids and finally the demands of the applications. . Results from multichannel pipette calibration can be reported for two or more individual channels. These choices are .

Whether you prefer mail-in or onsite pipette calibration, we offer a range of pipette calibrations that include ISO 8655 and ISO 17025-accredited pipette calibration to meet your regulatory needs. Rainin pipette service is rigorous and comprehensive, and it makes certain your pipettes deliver with the reliability and reproducibility your work .
This is why ISO 8655 is top standard for pipette calibration. ISO 8655 outlines extra requirements for the testing equipment, testing materials, and other environmental conditions. All elements of the testing procedures are recorded, and all the equipment is calibrated and verified too. Lab People has a fleet of trained service technicians and .Check the calibration of your pipettes regularly, depending on the frequency of use and on the application, but at least once a year. If daily . a three-month interval is recommended. Procedures to check calibration: The ISO 8655 specifications are defined to be used with the Forward pipetting technique, but by performing the adjustment and the— calibration laboratories, test houses, users of the equipment and other bodies as a basis for independent calibration, testing, verification and routine tests. . ISO 8655-6, ISO 8655-7 or ISO 8655-8. Any pipette so adjusted shall have clear, visible .
iso 8655 pipette standards
ISO 8655-2:2002, Piston-operated volumetric apparatus— Part 2: Piston pipettes ISO 8655-3:2002, Piston-operated volumetric apparatus— Part 3: Piston burettes ISO 8655-4:2002, Piston-operated volumetric apparatus— Part 4: Dilutors . NOTE 4 Calibration requires no operation which permanently modifies the apparatus.
ISO 8655 recognises pipettes, tips and service as a complete system. Pipette Service and Maintenance. 1. How long is the METTLER TOLEDO pipette performance warranty following service? . The frequency of both service and re-calibration is based on risk assessment. The risk assessment is likely to consider the frequency of the use of the .Whether you prefer mail-in or onsite pipette calibration, we offer a range of pipette calibrations that include ISO 8655 and ISO 17025-accredited pipette calibration to meet your regulatory needs. Rainin pipette service is rigorous and comprehensive, and it makes certain your pipettes deliver with the reliability and reproducibility your work .ISO 8655 is the official standard for piston-operated volumetric apparatuses, which includes pipettes, burettes, dilutors, dispensers, and manually operated precision laboratory syringes. An updated version of the standard came into force in May 2022 and included the following significant changes for pipettes:
Pipette manufacturers, who must provide an ISO 8655 calibration certificate with each pipette. Calibration results are only valid for the tip type used during the calibration, so if a pipette is .
Depending on the part of the ISO 8655 and the test type the requirements on the measurement procedure differ. The following requirements are given under the different parts of the ISO and for the different test types: ISO 8655-6 ISO 8655-7 ISO 8655-7 A.2 Test volumes Calibration and test At least at the following 3 volumes: --nominal volumeCalibration and adjustment of dispensing systems in the laboratory Abstract Userguide Kornelia Ewald, Eppendorf AG, Hamburg, Germany Introduction The test norm most commonly used for pipettes and dispensers and their calibration is the standard DIN EN ISO 8655. This standard lays down the maximum permissible ISO 8655:2022 defines two reference measurement procedures for pipette calibration: the gravimetric procedure (Part 6) and the photometric procedure (Part 8). Adhering to strict environmental conditions is crucial when using these reference procedures.Whether you prefer mail-in or onsite pipette calibration, we offer a range of pipette calibrations that include ISO 8655 and ISO 17025-accredited pipette calibration to meet your regulatory needs. Rainin pipette service is rigorous and comprehensive, and it makes certain your pipettes deliver with the reliability and reproducibility your work .
iso 8655 pipette specifications
Making us a full fledged custom composite autoclave manufacturer on top of our line of standard composite autoclaves. Bondtech’s composite autoclave systems also include key components in efficiency and precision that is expected from .
iso 8655 pipette calibration frequency|iso 8655 2022 pdf